
HL Paper 1
What is the correct explanation for the colour of [Cu(H2O)6]2+?
A. Light is absorbed when an electron moves to a d orbital of higher energy.
B. Light is released when an electron moves to a d orbital of higher energy.
C. Light is absorbed when electrons move from the ligands to the central metal ion.
D. Light is absorbed when electrons move between d and s orbitals.
Markscheme
A
Examiners report
What is the charge on the iron(III) complex ion in [Fe(OH)2(H2O)4]Br?
A. 0
B. 1+
C. 2+
D. 3+
Markscheme
B
Examiners report
The oxidation state of cobalt in the complex ion [Co(NH3)5Br]x is +3. Which of the following statements are correct?
I. The overall charge, x, of the complex ion is 2+.
II. The complex ion is octahedral.
III. The cobalt(III) ion has a half-filled d-subshell.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
[CoCl6]3– is orange while [Co(NH3)6]3+ is yellow. Which statement is correct?
A. [CoCl6]3– absorbs orange light.
B. The oxidation state of cobalt is different in each complex.
C. The different colours are due to the different charges on the complex.
D. The different ligands cause different splitting in the 3d orbitals.
Markscheme
D
Examiners report
Which of these statements are correct?
I. Zinc is not a transition element.
II. Ligands are Lewis bases.
III. Manganese(II) chloride is paramagnetic.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
This was one of only two questions where less than 50% of candidates got the correct answer. Many either didn't understand manganese chloride as paramagnetic or classified zinc as a transition element.
Which of these ions are likely to be paramagnetic?
I. Ti3+
II. Cr3+
III. Fe3+
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
Half of the candidates selected the correct combination of paramagnetic ions. The most commonly chosen distractor excluded Ti3+.
What is the overall charge, , of the chromium (III) complex?
A. 0
B. 1+
C. 2−
D. 3+
Markscheme
B
Examiners report
What is the oxidation state of the metal ion and charge of the complex ion in [Co(NH3)4Cl2]Cl?
Markscheme
C
Examiners report
This was one of the most challenging questions on the paper and it discriminated well between high scoring and low scoring candidates. 57 % of the candidates were able to use the formula of the compound to deduce the oxidation state of the metal ion and the charge of the complex ion. The most commonly chosen distractor was B where the charge of the complex ion was correct but the charge of the metal ion was not. Some teachers commented that the question was challenging but reasonable.
Which electrons are removed from iron (Z = 26) to form iron(II)?
A. two 3d electrons
B. two 4s electrons
C. one 4s electron and one 3d electron
D. two 4p electrons
Markscheme
B
Examiners report
Stating the 4s electrons are lost first in forming the Fe(II) ion was done correctly by 58 % but with a low discrimination index.
Which factor does not affect the colour of a complex ion?
A. temperature of the solution
B. identity of the ligand
C. identity of the metal
D. oxidation number of the metal
Markscheme
A
Examiners report
What is the effect of a stronger ligand?
Markscheme
A
Examiners report
Which is correct for the complex ion in [Fe(H2O)5Cl]SO4?
Markscheme
D
Examiners report
Why is hydrated copper (II) sulfate blue?
A. Blue light is emitted when electrons return to lower d-orbitals.
B. Light complimentary to blue is absorbed when electrons return to lower d-orbitals.
C. Blue light is emitted when electrons are promoted between d-orbitals.
D. Light complimentary to blue is absorbed when electrons are promoted between d-orbitals.
Markscheme
D
Examiners report
Higher scoring candidates managed to identify why hydrated copper(II) sulfate is blue in colour.
[Cr(OH2)6]3+ is violet and [Cr(NH3)6]3+ is yellow. What is correct?
The Colour Wheel
Markscheme
B
Examiners report
61% of the candidates were able to determine the relative d-level splitting and the wavelength of light absorbed by complex ions with different ligands, given the colours of the complex ions and a colour wheel labelled with the wavelengths of light.
Which complex has the greatest d orbital splitting?
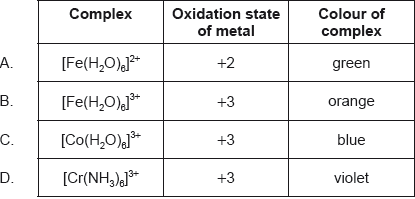
Markscheme
B
Examiners report
Which complex ion contains a central ion with an oxidation state of +3?
A. [PtCl6]2−
B. [Cu(H2O)4(OH)2]
C. [Ni(NH3)4(H2O)2]2+
D. [Co(NH3)4Cl2]+
Markscheme
D
Examiners report
Ammonia is a stronger ligand than water. Which is correct when concentrated aqueous ammonia solution is added to dilute aqueous copper(II) sulfate solution?
A. The d-orbitals in the copper ion split.
B. There is a smaller splitting of the d-orbitals.
C. Ammonia replaces water as a ligand.
D. The colour of the solution fades.
Markscheme
C
Examiners report
How is colour produced in transition metal complexes?
A. Light is absorbed when electrons are promoted between split d-orbitals.
B. Light is emitted when electrons fall between split d-orbitals.
C. Light is absorbed when electrons escape from the complex.
D. Light is emitted when the complex returns to ground state.
Markscheme
A
Examiners report
69 % of the candidates understood how colour is produced in transition metal complexes. The most commonly chosen distractor was B, which also recognized the involvement of the split d-orbitals, however stated that colour is produced when light is emitted when electrons fall between split d-orbitals.
Part of the spectrochemical series is shown for transition metal complexes.
I−< Cl− < H2O < NH3
Which statement can be correctly deduced from the series?
A. H2O increases the p–d separation more than Cl−.
B. H2O increases the d–d separation more than Cl−.
C. A complex with Cl− is more likely to be blue than that with NH3.
D. Complexes with water are always blue.
Markscheme
B