Tamoxifen
Tuesday 20 October 2015
A drug that has been used since the 1970s both for the prevention and for the treatment of certain types of breast cancer (in both women and men) is tamoxifen.

Some breast cancer cells require the female sex hormone oestrogen to grow. In the body tamoxifen is metabolised into a compound (4-hydroxytamoxifen) that binds to the oestrogen receptor site on the cancerous cells. This blocks the site and prevents the tumour cells from bonding with oestrogen so that they are unable to grow.
Tamoxifen has the following structure:
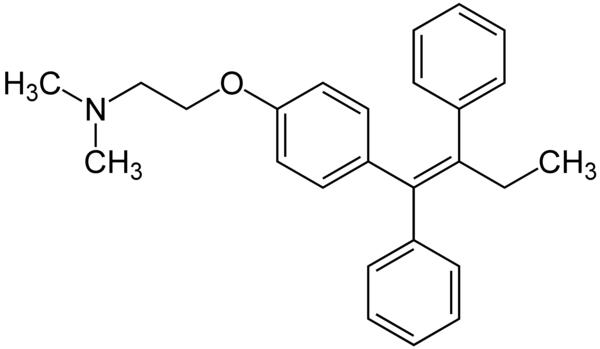
You could use the structure of tamoxifen to illustrate (or test) some of the syllabus content with your students.
For example:
(a) 10.2 Functional group chemistry: Identify four different functional groups contained within the molecule.
(Any four from: alkyl, ether, phenyl, alkenyl and amine)
(b) B.9 Biological pigments: Pedict whether tamoxifen is a white solid or exists as a coloured compound.
(There is considerable delocalisation of electrons so the energy required to promote an electron to an anti-bonding orbital may fall within the visible region of the spectrum rather than the ultra-violet. The colour that would then be seen would be the complementary colour to the colour absorbed. In fact tamoxifen is a white solid so it would appear that the amount of conjugation, although quite large, is still not sufficient to cause the absorption to fall in the visible region of the spectrum.)
(c) D.1 Pharmaceutical products and drug action: Suggest how the bioavailability of tamoxifen could be increased.
(Increase its solubility in water by converting the tertiary amine into a salt by reacting it with an acid. In fact tamoxifen is often administered as its citrate salt.)
(d) 20.3 Stereoisomerism: The IUPAC name for tamoxifen is (Z)-2-[4-(1,2-diphenylbut-1-enyl)phenoxy]-N,N-dimethylethanamine.
Explain the significance of the ‘(Z)’ in its name.
(Z gives information about the configurational isomerism of the molecule. Applying the Cahn-Ingold-Prelog (CIP) priority of the groups on each of the two carbon atoms in the double bond in the molecules it shows that the two highest groups lie on the same side. If they were on opposite sides it would be the E isomer).
Now it would appear that tamoxifen could also be used to illustrate another part of the syllabus. Sub-topic D. 2 in Option D covers penicillin and the fact that some ‘superbugs’, such as MRSA, are now completely resistant to antibiotics. A team from the School of Medicine and Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California in San Diego have just reported (13 October 2015) in Nature Communications that tamoxifen has been found to boost white blood cells making them more effective against bacterial diseases. When mice were treated with tamoxifen it considerably reduced mortality due to the ‘superbug’ MRSA. If this finding can be replicated in humans tamoxifen may prove to be an effective treatment against the antibiotic resistant methicillin-resistant staphylococcus aureus (MRSA) bacterium, which currently kills more than 5000 people a year in the USA alone.


Comments
To post comments you need to log in. If it is your first time you will need to subscribe.